ViSafe RedGel Stain - nucleic acid dye for staining DNA or RNA (delisted)
Suitable to stain dsDNA, ssDNA and RNA, as an alternative to Ethidium Bromide, GelRed or VIVA Sybrgreen Nucleic Acid Stain
delisted: Please see product S445: Gel Redsafe
Description:
ViSafe Red Gel Stain is designed to replace the highly toxic ethidium bromide (EtBr). Both red gel stain and EtBr have the same spectra, so the red gel stain able to replace the EtBr without changing existing imaging system. The dye is confirmed by Ames test results that it is impenetrable to latex gloves and cell membranes. By using the suggested working concentrations in gel staining, the red gel stain is proven unable to cross cell membranes; and it is noncytotoxic and nonmutagenic at working concentrations.
ViSafe Red Gel Stain, 10000X in H2O, can be diluted 10000X for precast gel protocol or 3000-3300X for post gel staining. One vial (0.5ml) of 10000X solution can be used for at least 100 minigels either using precast method or poststaining method.
Features:
- The red gel stain is non cytotoxic & non mutagenic shown by Ames
tests.
- It has a higher sensitivity More sensitive compared to EtBr or Viva
SybrGreen Nucleic Acid Stain.
- Extremely stable at room temperature for long-term storage.
Stable to be microwaved or being heated. The working solution is stable at room temperature when kept in dark. Wide application Suitable to stain dsDNA, ssDNA and RNA.
It is suitable to use in agarose gel or polyacrylamide gel and compatible with downstream applications, such as gel recovery & cloning.
Easy staining protocols and easy precast gel staining & post-staining procedures.
Compatible with most imaging system Gel can be viewed with standard UV transilluminator, visible light gel reader, or other gel imaging system.
Shipment: @ ambient temperature
Storage: 4-8°C or -20°C
How to use ViSafe RedGel stain I ?
Precast Protocol for 1% Agarose Gel
1. Pour 1g of agarose powder and 100ml 1X TAE or 1X TBE into glass
flask.
2. Melt the agarose in microwave for 1-3 mins until the agarose is
completely dissolved.
3. Add 3-5µl red gel stain per 100ml gel and stir the gel solution to mix
thoroughly.
Make sure the red gel stain is swirled and stirred well to mix with gel solution.
4. Pour the agarose solution onto gel plate and insert a comb.
5. Place newly poured gel at 4°C for 10-15 mins or stay at room
temperature for 20-30 mins, until it has completely solidified.
*Extra agarose containing the red gel stain can be kept in solid form at 4°C and can be remelted to cast more gels.
6. After the gel is ready, perform gel electrophoresis.
7. Visualize or image the gel directly under UV light or blue light after gel electrophoresis is done. Standard transilluminator or ethidium bromide filter can be used for gel imaging purpose.
Recommendations:
*Dilute the stock solution into agarose gel solution at 1:10000.
*Since the red gel stain is thermally stable, the stock solution can be
added while the gel solution is still hot.
*The red gel stain can be pre-combined with agarose powder and gel
working solution followed by microwaving or other heating procedures.
Remark:
- However, more dye might be necessary to be added for optimal signal.
- Agarose containing the red gel stain is not recommended to be stored in
molten form for more than a fewdays.
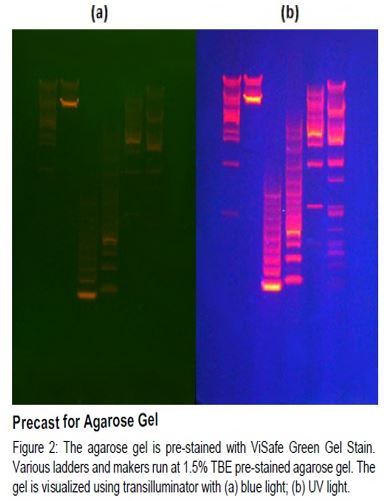
Remark:
Due to ViSafe Red Gel Stain’s slow diffusion rate in the relatively tight polyacrylamide gel matrix, the red gel stain is not suggested for staining DNA or RNA in precast poly-acrylamide gels.
- Precast staining method is not suitable for polyacrylamide gels.
Using precast gel with red gel stain is more conve-nient. However, the red gel stain can affect DNA migration duringstain is highly recommended. Pos t-s taining method may electrophoresis. Hence, post-staining with red gel lead t o gel results wi th higher s ensitivity and wi thout dye in ter ference wi th DNA migration. Besides, post -st ainingmethod is a simple protocol wi th no destaining and no s pecialbuffer needed.
The RedGel stain is non cytotoxic & non mutagenic shown by Ames
tests. It has a higher sensitivity More sensitive compared to EtBr or Viva
SybrGreen Nucleic Acid Stain. It is extremely stable Stable at room temperature for long-term storage.
* availibility of sample size may be limited
Final price excl. shipping costs3
- leider ausverkauft
How to use ViSafe RedGel stain II ?
Post-staining protocol for Agarose Gel and Polyacrylamide Gel
1. Run the gel electrophoresis accordingly.
2. Dilute 10000X ViSafe Red Gel Stain stock solutions to 3X staining solutions with 0.1M NaCl water solution. (This solution can be used at least 2-3 times, protected from light. Suggested to use container covered with alumin ium foil or use dark colour container.)
Example: 30µl of red gel stain added into 100ml of 0.1 M NaCl solution
*NaCl solution in the staining solution is optional. Adding NaCl in the staining solution enhances the staining, but may promote dye precipitation if the staining solution is to be used repeatedly. Any staining solution to be reuse d is preferably stored at room temperature in a dark pla ce to reduce possible dye precipitation problem.
3. Remove the gel from the gel tank and transfer into stain- ing container.
4. Allow the gel to stain for at least 25-30 mins with gentle shaking.
*Staining time may vary depending on the thickness of the gel and percentage of agarose.
5. Visualize or image the stained gel under UV light or blue light using standard transillumitor or ethidium bromide filter.
Recommendations:
*Higher content of acrylamide requires longer staining time. For polyacrylamide gels containing 3.5-10% acrylamide, typical staining time is 30mins to1hr.
*Destaining is not necessary, but rinsing the gel with water can reduce the background.
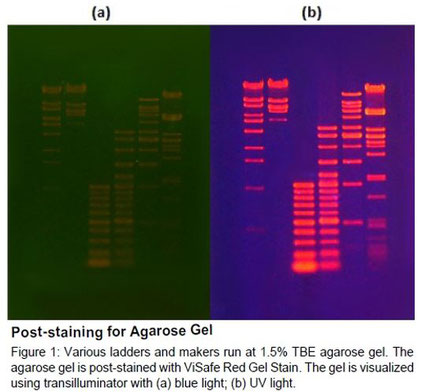
How to make it better?
1. To increase resolution of the DNA bands:
a. Running the gel at a lower voltage for longer period of time
b. Using a wider gel comb
c. Loading less DNA into well
2. To get better separation of bands, adjust the agarose percentage of the gel if the similarly sized bands that bands that are running too close together are loaded. A higher percentage agarose gel will help resolving smaller bands from each other, and a lower percentage gel will help separating larger bands.
3. ViSafe Red Gel Stain is efficiently removed from DNA by phenol/chloroform extraction and ethanol precipitation.
Related products
Deutsche Beschreibung

Datasheet VisSafe RedGel nucleic stain
tests.
- It has a higher sensitivity More sensitive compared to EtBr or Viva SybrGreen Nucleic Acid Stain.
Material Safety Datasheet

References / Protocols / Notes / Recomendations / Tests








