Buffy Coat Genomic DNA Kit - Magnetic Beads Based DNA Extraction Kit
This magnetic bead genomic DNA purification kit was designed specifically for isolating the genomic DNA from the Buffy Coat.
Magnetic Beads / Purification Kits / Extraction Kit / Isolation Kit / Buffy Coat
Description: Magnetic Buffy Genomic DNA isolation Kit
Description:
This magnetic bead genomic DNA purification kit was designed specifically for isolating the genomic DNA from the Buffy Coat. Its unique buffer system will efficiently lyse cells and degrade proteins, allowing for the DNA to be easily bound by the surface of the magnetic beads.
The RNA and other non-specific binding particles are removed with a wash buffer, and the genomic DNA is then released into the the Release Buffer. The genomic DNA can be purified manually within 10~15 minutes (using most magnetic separators) or the kit can be easily adapted to satisfy most automated nucleic acid purification systems.
Application
Sample: Up to 300 µl of the buffy coat
Operation time: 10~15 minutes (manual)
Applications: Restriction Enzyme Digestion, Southern Blotting, PCR and qPCR assays
Storage: Room temperature
Kit content:
Magnetic Beads - 2 ml
Lysis Buffer - 30 ml
Wash Buffer - 80 ml
Release Buffer - 20 ml
Final price excl. shipping costs3
- verfügbar / avaílable
- 3 - 5 days for delivery / 3 - 5 Tage Lieferzeit1
Troubleshooting
Refer to the table below to troubleshoot problems that you may encounter when purifying the
genomic DNA with the kit.
|
Problem |
Cause |
Solution |
|
DNA is sheared or degraded |
Lysate mixed too vigorously |
Use the appropriate pipette tip set for the required volume, lower it under the reading line of the solution to mix the sample, pipet up and down gently to mix. |
|
DNases contamination |
Maintain a sterile environment while working (e.g. wear gloves and use DNase-free reagents). |
|
|
RNA containment |
Incomplete removal of the RNAse |
RNase A treatment |
|
Low yields of gDNA |
Incomplete lysis and homogenization |
Complete lysis. Use the appropriate method for the lysate preparation based on the amount of starting materials. |
|
Incorrect handling of Magnetic Beads |
Vortex the tube containing the Magnetic Beads to fully resuspend the beads before adding them to your sample. |
|
|
Incorrect elution conditions |
Add the Release Buffer (50~100µl ) and incubate for 3 min at 65°C. |
|
|
The quality of the starting material may not be optimal |
Use fresh sample and process immediately after collection, or freeze the sample at -80°C or in the liquid nitrogen. |
|
|
High background on UV measurement |
Residual beads released |
Repeat the magnetic separation and transfer the eluate to a clean tube. |
Required Materials
- Magnetic separator
- 1.5 ml microcentrifuge tubes
- Water bath / Dry bath
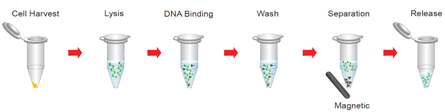
Protocol Magnetic Buffy Genomic DNA isolation Kit
Step 1. Lysis
1. Transfer up to 300 μl of the Buffy Coat into a 1.5 ml microcentrifuge tube and add 300 μl of the Lysis Buffer.
2. Mix well and incubate at 65°C for 5 minutes. During this time, pre-heat the Release Buffer to 65°C for the Step 4.
3. Add 300 μl of the absolute EtOH to the lysate and mix well.
Step 2. DNA Binding
1. Add 20 μl of Magnetic Beads. Mix well by gently shaking for 3 minutes.
2. Place the tube in a magnetic separator for 30 seconds.
3. Remove the solution (If the mixture becomes viscous, increase magnetic bead separation time)
Step 3. Wash
Add 800 μl of the Wash Buffer and mix well (Following the wash, the mixture will no longer be viscous).
2. Place the tube in a magnetic separator for 30 seconds. Remove the solution.
Step 4. Release
1. Add 200 μl of the Release Buffer (pre-heated to 65°C) and mix well.
2. Incubate for 3 minutes at 65°C (during the incubation, shake the tube vigorously every minute).
3. Place the tube in a magnetic separator for 1 minute.
4. Carefully transfer ONLY the clean portion of the solution to a clean tube.
NOTE: Be sure and allow the magnetic beads to disperse completely during the binding, wash and elution steps